| Word | Definition (or diagram/translation) |
|---|---|
| Particle Theory of Matter | Theory that describes the composition and behaviour of matter as being composed of small particles with empty space |
| Matter | Substance that has mass and occupies space |
| Mechanical Mixture | A heterogeneous mixture which one can physically separate |
| Suspension | A heterogeneous mixture where insoluble solid particles are distributed throughout a fluid, floating freely |
| Alloy | A combination of 2+ metals |
| Mixture | A substance that is made up of at least 2 types of particles |
| Qualitative property | A property of a substance that is not measured and doesn't have a numerical value, such as colour, odour, and texture |
| Quantative observation | An numerical observation |
| Precipitate | A solid that separates from a solution |
| Density | A measure of how much mass is contained in a given unit volume of a substance; calculated by dividing the mass of a sample of its volume (mass/volume) |
| Element | Element An element is made up of the same atoms throughout, and cannot be broken down further |
| Metal | a solid material that is typically hard, shiny, malleable, fusible, and ductile, with good electrical and thermal conductivity |
| Pure substance | A substance that is made up of only one type of particle |
| Atom | The smallest unit of matter found in substances |
| Solution | A uniform mixture of 2 or more substances |
| Colloid | is substance with small particles suspended in it, unable to be separated by gravity |
| Emulsion | A mixture of 2 insoluble liquids, in which one liquid is suspended in the other |
| Physical Property | Characteristic of a substance that can be determined without changing the makeup of the substance |
| Characteristic | A physical property that is unique to a substance and can be used to identify the substance |
| Periodic Table | a table of the chemical elements arranged in order of atomic number, usually in rows, so that elements with similar atomic structure (and hence similar chemical properties) appear in vertical columns. |
| Compound | Compounds are chemically joined atoms of different elements |
| Non-Metal | A substance that isn’t a metal |
| Physical Change | A change in which the composition of the substance remains unaltered and no new substances are produced |
| Chemical Change | A change in the starting substance and the production of ONE or more new substances Original substance does not disappear BUT the composition is rearranged |
| Molecule | Two or more non-metal atoms joined together |
| Diatomic Molecules | Molecules that only consists of 2 elements H O F BR I N C L - hyrodgen, oxygen, fluorine, bromine, iodine, nitrogen, chlorine. |
| Ions | A Charged particle, that results from a loss (cation - positve, less electrons) or gain (anion - negative, more electrons) of electrons when bonding |
| Electron | Negatively Charged |
| Proton | Positively Charged |
| Neutron | Neutral Charged | Ionic Charge | The sum of the positive and negative charges in a ion |
| Covalent Bond | The sharing of electrons between atoms when bonding |
| Valence Electrons | Number of electrons on the most outer orbit/shell of the element |
 |
| |
| |
|- Holds Shape
|
|- Holds Shape ### Sinking Or Floating?
- Most solids are **more** dense than liquids **(except water!)**
- When you place a solid object inside a liquid
- It will **sink** if the object is **more** dense than the liquid
- It will **float** if the object is **less** dense than the liquid
### Strange Behaviour of Water
- Its clear, odourless, tasteless, freezes at OoC, boils at 100oC
- `Its solid form floats on its liquid form!`
- `Water particles are different`
- Due to their `shape` and the way in which the `particles` are arranged, forces it to take up `more space` when packed.
- Its density is the highest at 4oC, where its in liquid form.
### Graphs
- To calculate the density on a graph, simply find the slope of any 2 points on the line in the graph
### Sinking Or Floating?
- Most solids are **more** dense than liquids **(except water!)**
- When you place a solid object inside a liquid
- It will **sink** if the object is **more** dense than the liquid
- It will **float** if the object is **less** dense than the liquid
### Strange Behaviour of Water
- Its clear, odourless, tasteless, freezes at OoC, boils at 100oC
- `Its solid form floats on its liquid form!`
- `Water particles are different`
- Due to their `shape` and the way in which the `particles` are arranged, forces it to take up `more space` when packed.
- Its density is the highest at 4oC, where its in liquid form.
### Graphs
- To calculate the density on a graph, simply find the slope of any 2 points on the line in the graph
 ## Quantitative Physical Properties
- **```Density```**: amount of ```stuff``` (or mass) per unit volume (g/cm3)
- **```Freezing Point```**: point where water solidifies (0oC)
- **```Melting Point```**: point where water liquefies (0oC)
- **```Boiling Point```**: point where liquid phase becomes gaseous (100oC)
## Common Qualitative Physical Properties
|Type|Definition|Example|
|:---|:---------|:------|
|Lustre|Shininess of dullness
## Quantitative Physical Properties
- **```Density```**: amount of ```stuff``` (or mass) per unit volume (g/cm3)
- **```Freezing Point```**: point where water solidifies (0oC)
- **```Melting Point```**: point where water liquefies (0oC)
- **```Boiling Point```**: point where liquid phase becomes gaseous (100oC)
## Common Qualitative Physical Properties
|Type|Definition|Example|
|:---|:---------|:------|
|Lustre|Shininess of dullness ## Properties Of Metals And Non-Metals
|Type|Properties|Picture|
|:---|:---------|:------|
|Metals|- Metals are good `conductors` of heat and electricity
## Properties Of Metals And Non-Metals
|Type|Properties|Picture|
|:---|:---------|:------|
|Metals|- Metals are good `conductors` of heat and electricity |
|Non-Metals|- Non-metals are poor conductors of heat and electricity
|
|Non-Metals|- Non-metals are poor conductors of heat and electricity |
|Metalloids|- Metalloids (metal-like) have properties of both metals and non-metals
|
|Metalloids|- Metalloids (metal-like) have properties of both metals and non-metals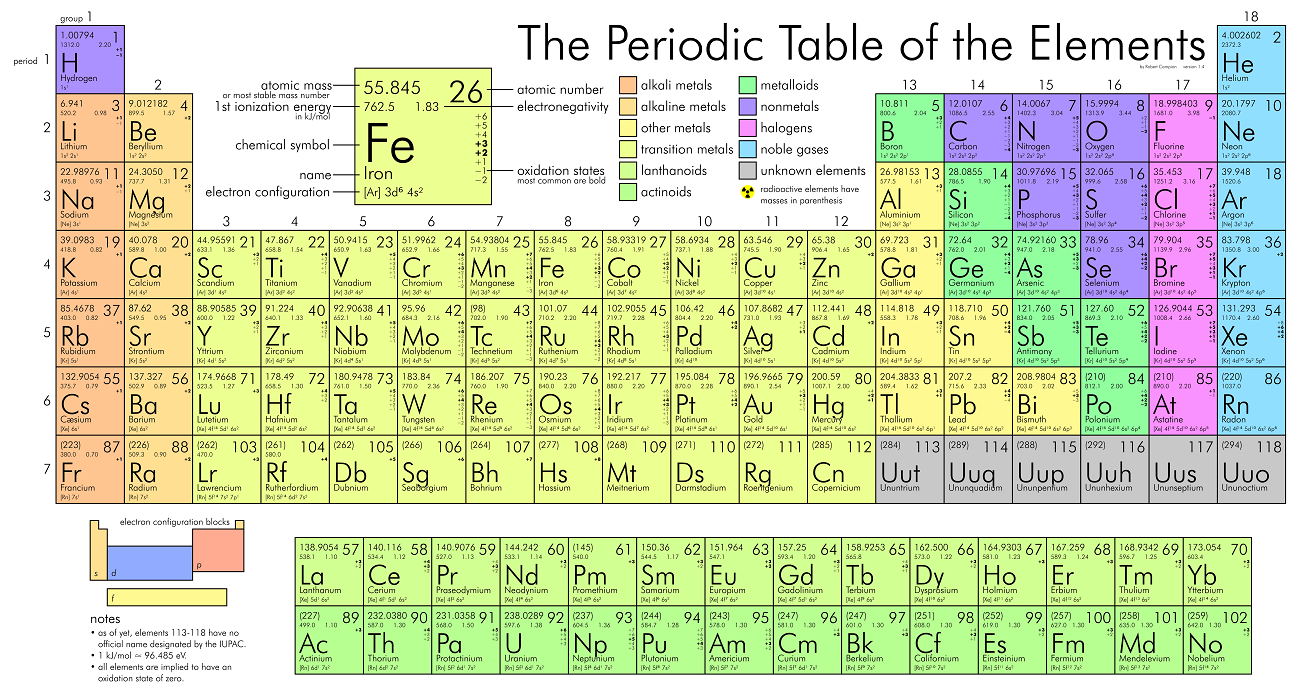 ### Trends On The Periodic Table
- The first column are the `Alkali metals`.
- They are shiny, have the consitency of clay, and are easily cut with a knife.
- They are the **most reactive** metals.
- They react violently with water.
- Alkali metals are **never found as free elements in nature**. They are always bonded with another element.
- The second column are the `Alkaline earth metals`.
- They are **never found uncombined in nature**.
- The last column are the `Noble gases`.
- **Extremely un-reactive**.
- The second last column are the `Halogens`.
- The **most reactive non-metals**
- They **react with alkali metals to form salts**.
- The middle parts are the `transition metals`.
- They are good conductors of heat and electricity.
- Usually bright coloured.
- They have properties similar to elements in their same family
- Many of them combine with oxygen to form compounds called oxides.
- The rows outside the table are the `Inner tranistion metals`.
### Trends On The Periodic Table
- The first column are the `Alkali metals`.
- They are shiny, have the consitency of clay, and are easily cut with a knife.
- They are the **most reactive** metals.
- They react violently with water.
- Alkali metals are **never found as free elements in nature**. They are always bonded with another element.
- The second column are the `Alkaline earth metals`.
- They are **never found uncombined in nature**.
- The last column are the `Noble gases`.
- **Extremely un-reactive**.
- The second last column are the `Halogens`.
- The **most reactive non-metals**
- They **react with alkali metals to form salts**.
- The middle parts are the `transition metals`.
- They are good conductors of heat and electricity.
- Usually bright coloured.
- They have properties similar to elements in their same family
- Many of them combine with oxygen to form compounds called oxides.
- The rows outside the table are the `Inner tranistion metals`.
 - The **left** to the **staircase** are the metals and the **right** are the non-metals. The ones touching the **staircase** are the `metalloids`.
- The **left** to the **staircase** are the metals and the **right** are the non-metals. The ones touching the **staircase** are the `metalloids`.
 ### How To Read An Element
### How To Read An Element
 ## History of The Atom
|Person|Description|Picture|
|:-----|:----------|:------|
|Democritus|All matter can be divided up into smaller pieces until it reaches an unbreakable particle called an ATOM (cannot be cut)
## History of The Atom
|Person|Description|Picture|
|:-----|:----------|:------|
|Democritus|All matter can be divided up into smaller pieces until it reaches an unbreakable particle called an ATOM (cannot be cut) |
|John Dalton|- Billbard model, atoms of **different elements are different**
|
|John Dalton|- Billbard model, atoms of **different elements are different** |
| |
|Ernest Rutherford|- Discovered that the postively charged **nucleus**.
|
|Ernest Rutherford|- Discovered that the postively charged **nucleus**. 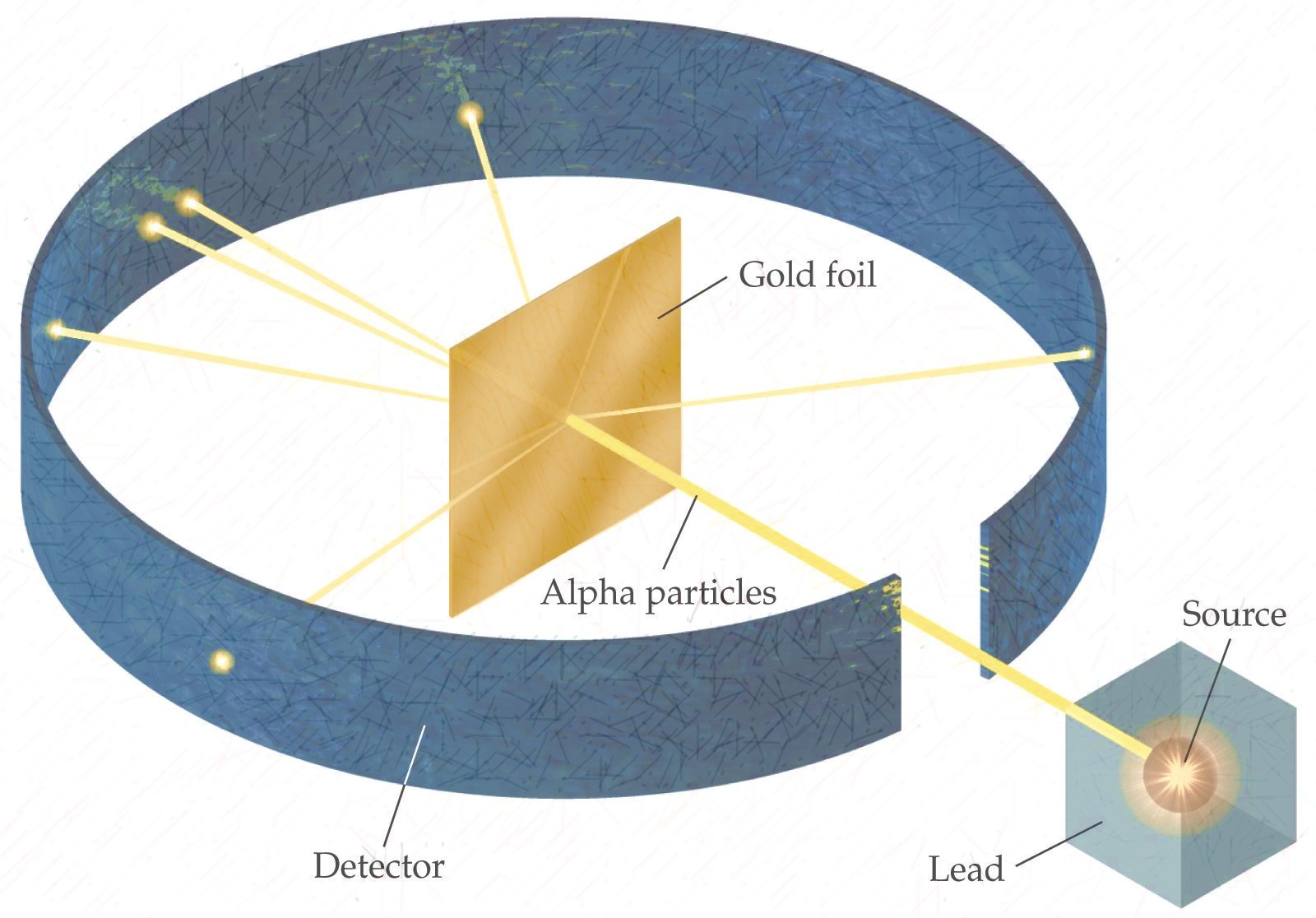 |
|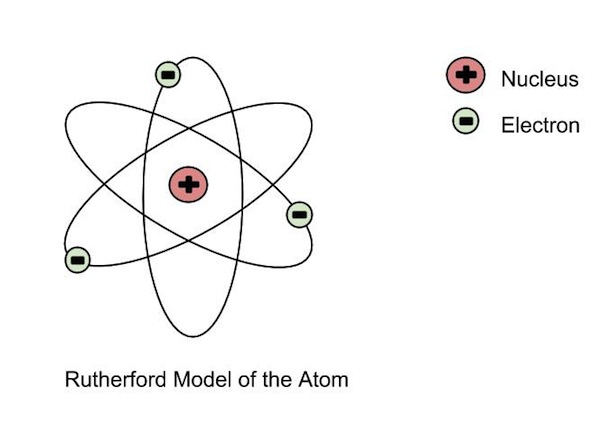 |
|Niels Bohr|- Discovered that electrons **orbit the nucleus in fixed paths**, each electron has a **definite** amount of energy, further from nucleus = more energy.
|
|Niels Bohr|- Discovered that electrons **orbit the nucleus in fixed paths**, each electron has a **definite** amount of energy, further from nucleus = more energy. |
|James Chadwick|- Discovered the neutron, mass of neutron = mass of proton (basically)
|
|James Chadwick|- Discovered the neutron, mass of neutron = mass of proton (basically) |
## Carbon
## Atoms
- Subscripts - tells us how many of the atom are there, for example N2 means there are 2 nitrongen atoms.
- Use distrubutive property if there are brackets and a subscript, for example, (CO)2 is equilivant to C2O2.
- Atoms are stable if they have a full valence shell (noble gases)
- Each family has the same amount of valence electrons as their family number, so `alkali metals` would have 1 valence electron, `alkaline earth metals` will have 2, `halogens will have` 7 and `noble gases` would have 8.
- They will also have the same amount of protons as their `atomic number`.
- **Number of protons = Number of electrons**.
- **Number of neutrons = mass - atomic number/number of protons**.
## Bohr-Rutherford / Lewis-Dot Diagrams
- **Bohr-Rutherford**
- Draw nucleus, and draw the apprioate number of orbits.
- Put number of **protons** and **neutrons** in the nucleus.
- Draw the correct number of electrons in each orbit
|
## Carbon
## Atoms
- Subscripts - tells us how many of the atom are there, for example N2 means there are 2 nitrongen atoms.
- Use distrubutive property if there are brackets and a subscript, for example, (CO)2 is equilivant to C2O2.
- Atoms are stable if they have a full valence shell (noble gases)
- Each family has the same amount of valence electrons as their family number, so `alkali metals` would have 1 valence electron, `alkaline earth metals` will have 2, `halogens will have` 7 and `noble gases` would have 8.
- They will also have the same amount of protons as their `atomic number`.
- **Number of protons = Number of electrons**.
- **Number of neutrons = mass - atomic number/number of protons**.
## Bohr-Rutherford / Lewis-Dot Diagrams
- **Bohr-Rutherford**
- Draw nucleus, and draw the apprioate number of orbits.
- Put number of **protons** and **neutrons** in the nucleus.
- Draw the correct number of electrons in each orbit
 - **Lewis-Dot Diagrams**
- Draw element symbol
- Put the right number of valence electrons around the symbol, perferably in pairs
- **Lewis-Dot Diagrams**
- Draw element symbol
- Put the right number of valence electrons around the symbol, perferably in pairs
 ### Bonding
- To combine 2 atoms, each element wants to be stable. So they each want a full valence shell, (outer shell) so they are stable.
- They can either `gain`, `lose` or `share` electrons in order to become stable.
- Example:
- Oxygen and Hydrogen, in order to become stable, they all need 8 valence electrons. Hydrogen has 1, oxygen has 6, so we bring in another hyrdogen and we let them share all their electrons, turning into H2O, or water.
### Bonding
- To combine 2 atoms, each element wants to be stable. So they each want a full valence shell, (outer shell) so they are stable.
- They can either `gain`, `lose` or `share` electrons in order to become stable.
- Example:
- Oxygen and Hydrogen, in order to become stable, they all need 8 valence electrons. Hydrogen has 1, oxygen has 6, so we bring in another hyrdogen and we let them share all their electrons, turning into H2O, or water.
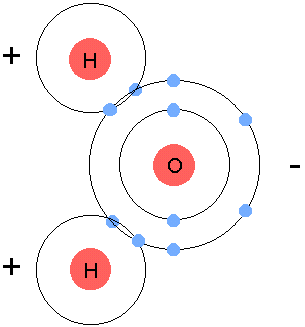 - Use **arrows** to show gaining or losing electrons.
- **Circle** to show sharing of electrons.
## Naming of Ionic Bonds
1. Write cation (metal) first
2. Write anion (non-metal) second
3. Change the ending of the non-metal to ```ide```.
- Use **arrows** to show gaining or losing electrons.
- **Circle** to show sharing of electrons.
## Naming of Ionic Bonds
1. Write cation (metal) first
2. Write anion (non-metal) second
3. Change the ending of the non-metal to ```ide```.

 ## Decomposition
- A chemical change used to break compounds down into simpler substances
- Energy must be ADDED
- Using electricity
- Adding thermal energy
## Catalyst
- Substance that accelerates a chemical change without being consumed OR changed itself
## Uses of Hydrogen Peroxide
- On cuts/scraps
- Blood has a catalyst = see bubbling O2
- Cleans contact lenses
- Bubbling removes dirt
- Bleaches
- React with compounds that provide color
- RESULT = no colour (bleach blond hair/teeth)
## Decomposition
- A chemical change used to break compounds down into simpler substances
- Energy must be ADDED
- Using electricity
- Adding thermal energy
## Catalyst
- Substance that accelerates a chemical change without being consumed OR changed itself
## Uses of Hydrogen Peroxide
- On cuts/scraps
- Blood has a catalyst = see bubbling O2
- Cleans contact lenses
- Bubbling removes dirt
- Bleaches
- React with compounds that provide color
- RESULT = no colour (bleach blond hair/teeth)